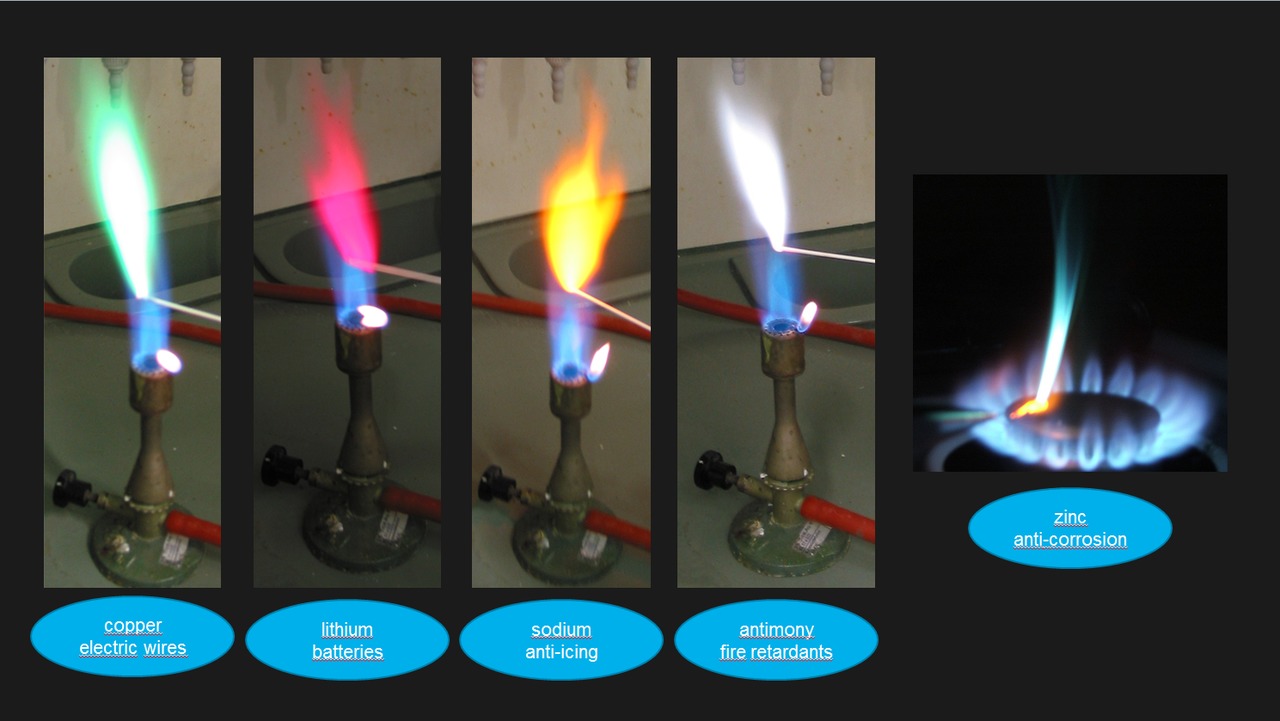
Copper Oxide Flame Color . this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. For example, copper (i) emits blue light. the flame test also can distinguish between the oxidation states of atoms of a single element. 50 rows the flame test carried out on a copper halide. The color can be used to detect halides by using. it's a green fireball! different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. When copper atoms are heated their electrons. the copper flame color is dependent on the presence of halide (i, f, br, or cl). Flame tests are used to identify.
from ar.inspiredpencil.com
When copper atoms are heated their electrons. Flame tests are used to identify. it's a green fireball! The color can be used to detect halides by using. For example, copper (i) emits blue light. different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. 50 rows the flame test carried out on a copper halide. the flame test also can distinguish between the oxidation states of atoms of a single element. the copper flame color is dependent on the presence of halide (i, f, br, or cl). this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises.
Copper Flame Color
Copper Oxide Flame Color it's a green fireball! different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. the copper flame color is dependent on the presence of halide (i, f, br, or cl). it's a green fireball! When copper atoms are heated their electrons. For example, copper (i) emits blue light. Flame tests are used to identify. 50 rows the flame test carried out on a copper halide. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the flame test also can distinguish between the oxidation states of atoms of a single element. The color can be used to detect halides by using.
From sbornik-fraz.ru
Сколько весит огонь загадка Copper Oxide Flame Color the flame test also can distinguish between the oxidation states of atoms of a single element. For example, copper (i) emits blue light. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. different metal electrons emit different wavelengths of light to return to. Copper Oxide Flame Color.
From tedkinsman.photoshelter.com
Copper(II) Chloride Flame Test Copper Oxide Flame Color the flame test also can distinguish between the oxidation states of atoms of a single element. When copper atoms are heated their electrons. the copper flame color is dependent on the presence of halide (i, f, br, or cl). 50 rows the flame test carried out on a copper halide. different metal electrons emit different wavelengths. Copper Oxide Flame Color.
From pngtree.com
Fire Flame Particle Transparent Clipart, Fire, Flame, Light PNG Copper Oxide Flame Color different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. it's a green fireball! this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The color can be used to detect halides by. Copper Oxide Flame Color.
From www.poolwarehouse.com
Brazilian Flame 3 Skewer Rotisserie Brazilian Gas Grill Copper Oxide Flame Color different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. For example, copper (i) emits blue light. When copper atoms are heated their electrons. it's a green fireball! this page describes how to perform a flame test for a range of metal ions, and briefly discusses. Copper Oxide Flame Color.
From exoirroix.blob.core.windows.net
How To Remove Nitrous Oxide From The Atmosphere at Mallory McLaughlin blog Copper Oxide Flame Color it's a green fireball! the flame test also can distinguish between the oxidation states of atoms of a single element. The color can be used to detect halides by using. different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. this page describes how to. Copper Oxide Flame Color.
From modrinth.com
vKnitOn Modrinth Copper Oxide Flame Color For example, copper (i) emits blue light. The color can be used to detect halides by using. 50 rows the flame test carried out on a copper halide. it's a green fireball! this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the. Copper Oxide Flame Color.
From edurev.in
Chemical Properties of Metals and NonMetals Class 10 Notes EduRev Copper Oxide Flame Color For example, copper (i) emits blue light. Flame tests are used to identify. the copper flame color is dependent on the presence of halide (i, f, br, or cl). it's a green fireball! this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The. Copper Oxide Flame Color.
From classlibraryfruehauf.z19.web.core.windows.net
Propane Flame Color Chart Copper Oxide Flame Color The color can be used to detect halides by using. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. For example, copper (i) emits blue light. When copper atoms are heated their electrons. the flame test also can distinguish between the oxidation states of. Copper Oxide Flame Color.
From www.zerochan.net
Charizard Pokémon Image by GENZOMAN 3904195 Zerochan Anime Image Copper Oxide Flame Color it's a green fireball! The color can be used to detect halides by using. Flame tests are used to identify. For example, copper (i) emits blue light. the flame test also can distinguish between the oxidation states of atoms of a single element. When copper atoms are heated their electrons. 50 rows the flame test carried out. Copper Oxide Flame Color.
From www.pinterest.co.uk
Flame Test Colors and Procedure (Chemistry) Flame test, Chemistry Copper Oxide Flame Color this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. 50 rows the flame test carried out on a copper halide. The color can be used to detect halides by using. the flame test also can distinguish between the oxidation states of atoms of. Copper Oxide Flame Color.
From www.ouestny.com
What is a flame in chemistry? Copper Oxide Flame Color this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the copper flame color is dependent on the presence of halide (i, f, br, or cl). Flame tests are used to identify. 50 rows the flame test carried out on a copper halide. When. Copper Oxide Flame Color.
From www.educationusingpowerpoint.co.uk
AQA Chemistry Unit 3 Education Using Powerpoint Copper Oxide Flame Color this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. For example, copper (i) emits blue light. different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. 50 rows the flame test carried. Copper Oxide Flame Color.
From www.bulbapp.io
The Chemical Connection between Fluorine and Colored Flames [EN/TR] BULB Copper Oxide Flame Color this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the flame test also can distinguish between the oxidation states of atoms of a single element. The color can be used to detect halides by using. the copper flame color is dependent on the. Copper Oxide Flame Color.
From kvepalusala.lt
Inebrya Color 100ml plaukų dažai Copper Oxide Flame Color the copper flame color is dependent on the presence of halide (i, f, br, or cl). this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Flame tests are used to identify. When copper atoms are heated their electrons. The color can be used to. Copper Oxide Flame Color.
From www.zerochan.net
Hazbin Hotel Image by luchokme 3864606 Zerochan Anime Image Board Copper Oxide Flame Color When copper atoms are heated their electrons. Flame tests are used to identify. the copper flame color is dependent on the presence of halide (i, f, br, or cl). The color can be used to detect halides by using. the flame test also can distinguish between the oxidation states of atoms of a single element. For example, copper. Copper Oxide Flame Color.
From dxoqbloyt.blob.core.windows.net
Flame Test Lab The Identification Of An Element Answers at Linda Copper Oxide Flame Color For example, copper (i) emits blue light. different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. the copper flame color is dependent on the presence of halide (i, f, br, or cl). 50 rows the flame test carried out on a copper halide. it's. Copper Oxide Flame Color.
From worksheetfullsyncarpy.z14.web.core.windows.net
Procedure Of Flame Test Copper Oxide Flame Color For example, copper (i) emits blue light. When copper atoms are heated their electrons. different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. it's a green fireball! Flame tests are used to identify. the flame test also can distinguish between the oxidation states of atoms. Copper Oxide Flame Color.
From www.pinterest.co.uk
Effect of anion on flame colours of metal salts Chemistry classroom Copper Oxide Flame Color For example, copper (i) emits blue light. different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. When copper atoms are heated their electrons. the copper flame color is dependent on the presence of halide (i, f, br, or cl). the flame test also can distinguish. Copper Oxide Flame Color.